Infectious Disease > HAV IgM Rapid Test Kit >
HAV IgM Rapid Test Kit
INTENDED USE
The HAV IgM One Step Rapid Test is a lateral flow chromatographic immunoassay for the qualitative detection of IgM antibody to Hepatitis A virus (HAV) in human serum or plasma. It is intended to be used as a screening test and as an aid in the diagnosis of infection with HAV. Any reactive specimen with the HAV IgM Rapid Test must be confirmed with alternative testing method(s) and clinical findings.
PRINCIPLE
The HAV IgM Rapid Test is a lateral flow chromatographic immunoassay. The test cassette consists of: 1) a burgundy colored conjugate pad containing mouse anti-human IgM antibody conjugated with colloid gold (IgM conjugates) and, 2) a nitrocellulose membrane strip containing a test band (T band) and a control band (C band). The T band is pre-coated with recombinant HAV antigen, and the C band is pre-coated with goat anti-mouse IgM antibodies.
When an adequate volume of test specimen is dispensed into the sample well of the cassette, the specimen migrates by capillary action across the cassette. Anti-HAV IgM if present in the specimen will bind to the IgM conjugates. The immunocomplex is then captured on the membrane by the pre-coated HAV antigen, forming a burgundy colored T band, indicating a HAV IgM positive test result.
Absence of the T band suggests a negative result. The test contains an internal control (C band) which should exhibit a burgundy colored band of the immunocomplex of goat anti-mouse IgG/ IgM-gold conjugate regardless of the color development on the T band. Otherwise, the test result is invalid and the specimen must be retested with another device.
ASSAY PROCEDUREASSAY PROCEDURE
Step 1: Bring the specimen and test components to room temperature if refrigerated or frozen. Mix the specimen well prior to assay once thawed.
Step 2: When ready to test, open the pouch at the notch and remove device. Place the test device on a clean, flat surface.
Step 3: Be sure to label the device with specimen’s ID number.
Step 4: Fill the pipette dropper with the specimen. Holding the dropper vertically, dispense 1 drop (about 30-45μL) of specimen into the sample well making sure that there are no air bubbles. Then add 1 drop (about 35-50μL) of Sample Diluen immediately.
Step 5: Set up timer.
Step 6: Results can be read in 15 minutes. Positive results can be visible in as short as 1 minute.
Don’t read result after 15 minutes. To avoid confusion, discard the test device after interpreting the result.
INTERPRETATION OF ASSAY RESULT


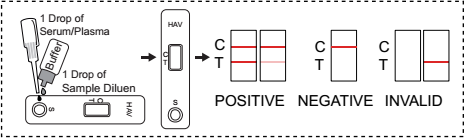
Positive: If both C and T band are developed, the test indicates for the presence of IgM anti-HAV in the specimen. The result is positive.
Negative: If only the C band is developed, the test indicates that no detectable IgM anti-HAV is present in the specimen. The result is negative.
Invalid: Control line fails to appear.
Categories
Contact Us
Tel:+86-13514318398
Fax :+86-4000068521
Mobile:+86-13514318398
E-mail: sales@mdmedicalproduct.com
Skype: fionazheng2011
Whatsapp: +86-13514318398